Intestinal delivery with enhanced bioavailability is of great significance for stomach irritating and pungent bioactive molecules like Capsaicinoids from red chili peppers. Because, oral delivery is the one and only approved and preferred route of administration for nutrients (Nutraceuticals and Functional foods). But, there exist many challenges for pungent capsaicinoids. High dosage due to poor bioavailability, unfavorable organoleptic properties (pungency, bitterness, and aroma), gastrointestinal discomforts (heart burn, borborygmus, vomiting, stomach irritation, gastritis, abdominal pain, and ulceration) etc are the major concerns. Though some attempts have been reported using the principles of self-emulsifying drug delivery (SMEDDS), they are useful only for topical applications like pain balms, owing to the liquid state, lack of pungency masking, and excessive use of pharma grade synthetic emulsifiers. Here comes the necessity of powder forms suitable for the oral delivery formulations, especially in a 100% natural, food-grade and clean label format for Functional food and Nutraceuticals.
AKAY’s FENUMAT technology has already received greater attention as a 100% Natural GREEN platform delivery technology for the delivery of lipophilic phytonutrients like curcumin, boswellic acids etc, either alone or as a co-delivery single powder form with enhanced bioavailability for each molecule (Journal of Functional Foods, 2021 and 2016). FENUMAT is leading the way for next generation nutraceuticals, which are natural, clean and food-grade. So, we applied our FENUMAT technology to pungent red chili extracts with a view to develop pungency-masked food-grade micro-beadlets (Capsifen) for the sustained-intestinal delivery with enhanced bioavailability, mentioned the Chief Research Officer at Akay, Dr. Krishnakumar.
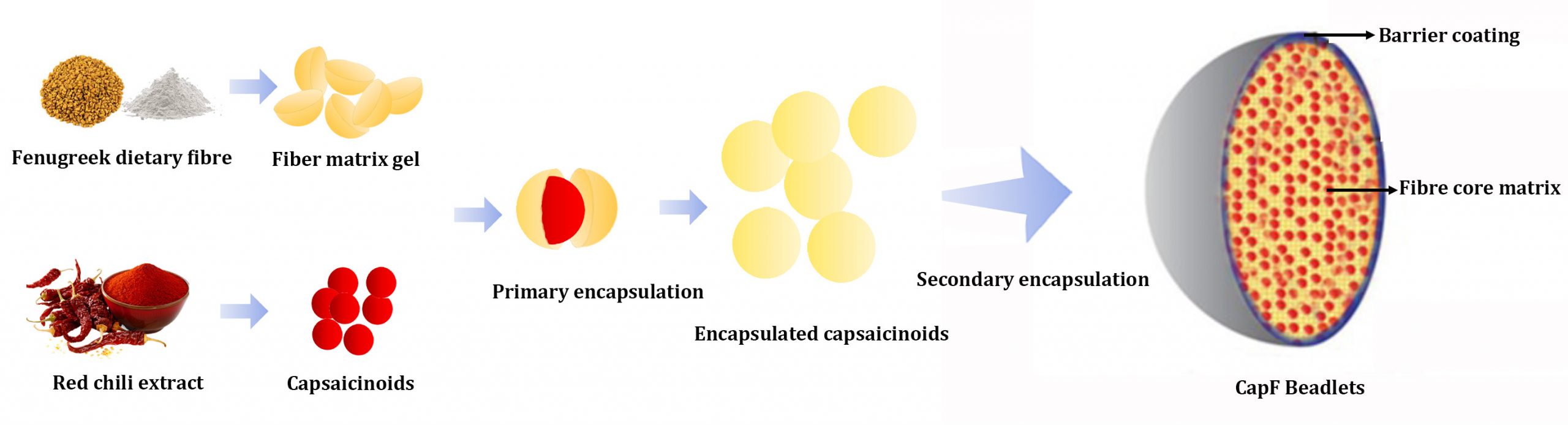
In the study, the authors received uniform microbeads (250 to 400 µm) containing not less than 2 or 3% capsaicinoids content from Akay Natural Ingredients, Cochin, India which are manufactured in their GMP-facility using a proprietary gel-phase homogenization technique followed by Marmurisation and Fluid bed coating. The study characterised the Capsifen beadlets as stable, amorphous, directly compressible, and free flowing microbeads with a tap density of > 0.65 g/mL, following a battery of the state-of-the-art characterisation techniques such as NMR, FTIR, DSC, PXRD, DLS and SEM analysis.
Swelling studies, in vitro release kinetics and particle size analysis indicated the sustained-intestinal delivery of soluble capsaicinoids of about 100 ± 10 nm for better absorption, without pungency discomforts. Further pharmacokinetic studies on Wistar rats revealed enhanced bioavailability (19-fold) of Capsifen compared with that of unformulated capsaicinoids. Capsifen was safe upon gastric mucosa irritation test and demonstrated significant (p < 0.05) anti-obesity effect in high fat diet-induced hypercholesteremic rats.
Capsifen opens up a new arena to the formulators and Nutraceutical manufacturers to make utilise the remarkable benefits of red chili capsaicinoids, with 100% Natural and Vegan claims. Clean label and Vegan beadlets for red chili capsaicinoids with proper scientific studies and patented technology is a long time pending requirement of the industry. Thanks to our R&D who developed this great formulation for us…Go Green, Go Natural…. added Mr. Emmanuel, Chief Marketing officer at Akay. Now capsifen can provide Vegan beadlets to consumers as a pre-work out agent for their body weight management and to burn the calories naturally. Human safety, tolerance and efficacy staudy of capsifen has already been published. Another clinical on the influence of Capsifen on Thermogensis is going on, with encouraging initial results. …he added.
The consumers are aware of the potential health benefits of red chili peppers. Various preclinical and clinical studies have also demonstrated the benefits of capsaicinoids. For instance, capsaicinoids reduce adiposity by enhancing the energy expenditure and fat metabolism through increased catecholamine secretion. Properties like thermogenesis, lipid metabolism, carbohydrate oxidation, and reduction in the cumulative ad libitum energy and food intake makes them novel for weight management and energy burning. Under conditions of obesity, it inhibits adipokine release, and macrophage activation to release pro-inflammatory mediators. Capsaicin has also been shown to offer protection and pain relief (analgesic) against a variety of inflammatory and autoimmune conditions, including arthritis and nerve damage.
Despite the above-mentioned health beneficial pharmacological effects, poor oral bioavailability, short half-life, and the lack of safe oral delivery forms limit the functional and therapeutic use of capsaicinoids. Capsaicinoids are rapidly metabolized in the liver. Chaiyasit et al., (2009) have reported that the consumption of 5 g of C. frutescens extract as soft gel capsule resulted in a peak plasma concentration of only 2.5 ng/mL at 45 minutes and no capsaicin was detected after 105 min. Above all, its burning effect and stinging pain to the skin. If ingested in excess, it may produce damage to the inner linings of the gastrointestinal tract leading to abdominal pain, acidity, nausea, and palpitations. Handling difficulties such as upper respiratory tract discomforts and skin allergy have also been accounted as the issues, which limit its utilization. Here comes the potentiality of Capsifen uniform beadlets with enhanced bioavailability and no handling issues. You can even put the Capsifen powder directly into your tongue….!!! Claims Dr. Krishnakumar.
Reference: A Joseph et al. Journal of Functional Foods 85 (2021); A green approach for the sustained intestinal delivery of red chili (capsicum annum L) extracted capsaicinoids with enhanced bioavailability.